Myco-Read™ Mycoplasma Detection Kit (PCR)
- Home
- Life Science
- Myco-Read™ Mycoplasma Detection Kit (PCR)
상품 정보
상품 상세설명
Description
One of the most concerning factors in cell culture is microorganism infection. When infected with yeast, fungi, or bacteria, there are visible features. Mycoplasma contamination, however, is not visible by general microscopic observation and does not have significant features to confirm infection.
Various methods have been used to detect mycoplasmas.
They can be roughly classified into the culture method, enzyme-based method, or PCR-based method. In the case of the culture method, mycoplasma must be inoculated into solid culture medium to confirm colony formation, which is much costly and time consuming than other microorganisms. Enzyme-based methods require also expensive equipment and reagents. The PCR method uses 16s rRNA, which is the unique biological marker for species classification. Using the characteristics of these rRNAs, PCR can be performed using the primers of the 16S rRNA sequences of the species to be classified. This PCR method has advantages over other methods in terms of time, cost, and sensitivity.
Myco-Read™ Mycoplasma Detection Kit (PCR) is a product that can detect mycoplasma infections based on PCR. This product does not react with other microorganism since the primers were designed to be specific to mycoplasma 16S rRNA. In addition, this kit contains internal control DNA to avoid false negative results.
Kit contents
| Components | SMD0172 |
SMD0173 |
Storage |
|---|---|---|---|
2x PCR Master Mix | 500 ㎕ |
1000 ㎕ |
-20 ℃ |
| Primer Set | 100 ㎕ | 200 ㎕ | |
| Positive Control | 50 ㎕ | 100 ㎕ | |
| Ultra Pure Water |
500 ㎕ | 1000 ㎕ |
Technical data
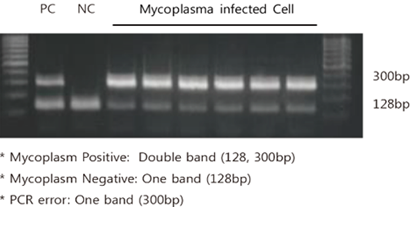
Internal control DNA was detected at 128 bp and mycoplasma at 300 bp respectively. Therefore, the infected samples have a double band of 128 and 300 bp. The non-infected sample only has a 128 bp band.



