Myco-Guard™ PLUS Mycoplasma Elimination Reagent
- Home
- Life Science
- Myco-Guard™ PLUS Mycoplasma Elimination Reagent
상품 정보
상품 상세설명
Description
The mycoplasmas are characterized by their lack of a rigid cell wall. Mycoplasmas are considered to be the smallest self-replicating organisms. Their small 0.3-0.8㎛ diameter and flexible cell membrane allow them to pass through commonly used anti-bacteriological filters with a diameter of 0.45㎛. Because of these distinct biological features, mycoplasma-contaminated cultures and infected cells cannot be eliminated with common antibiotics.
According to academic reports, about 80% of all cultured cell lines are infected with mycoplasma. Mycoplasma contaminations have a significant impact on research results and product quality at research facilities, pharmaceutical companies, and biological product plants.
Unlike other microorganisms such as bacteria or fungi, the effect of mycoplasma on cell growth may not be detectable by the naked eye. However, mycoplasma contamination affects DNA and RNA protein synthesis in cells and results in the transformation of chromosomes and plasma membrane antigens.
Myco-Guard™ PLUS uses a combination of antibiotics to effectively eliminate a variety of mycoplasma species as well as mycoplasma that is resistant to a majority of anti-mycoplasma agents. Each antibiotic removes mycoplasma by inhibiting protein synthesis through its own mechanism. These mechanisms act on mycoplasma and Gram (+) bacteria but do not act on eukaryotic cells. The cytotoxicity of Myco-Guard™ PLUS is very low. When used in cultured cells, cell growth may be slowed; however, after Myco-Guard™ PLUS is removed, the cell growth rate restores to normal.
Kit Contents
| Cat No. | Components | Size | Concentration | Storage |
|---|---|---|---|---|
| SMD022P | Myco-Guard™ PLUS Mycoplasma Elimination Reagent |
0.5 ㎖ x 4 vials | 50 ㎎/㎖ | -20 ℃ |
Technical data
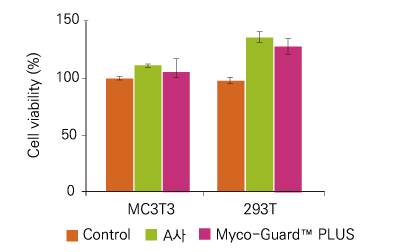
Figure 1. Cytotoxicity Test using Quanti-Max™. The cells were seeded at 1x104 cells/well in 96-well plate and incubated 24 hours at 37℃. The Myco-Guard™ was then added into the plate. And cell viability assays were performed according to the Quanti-Max™ assay protocol.
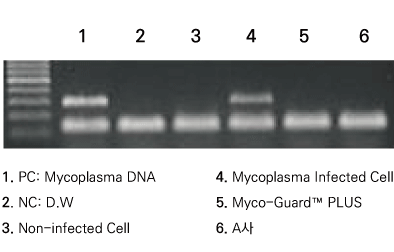
Figure 2. Detection performance for mycoplasma contamination. Mycoplasma is elimination in Myco-Guard™ treatment group.
Procedure
1. Concentration for Elimination: 500X ~ 2,000X
a. According to cell status, it should be adjusted to amount of Myco-Guard™ PLUS.
b. If the cells are in a severe infection state, the cell condition is not very good. So in this situation, concentration of Myco-Guard™ PLUS is recommended to 2,000X level
| Concentration | T 25 flask (5 ㎖) | T75 flask (15㎖) | 500 ㎖ Media Bottle |
|---|---|---|---|
| 2,000X (25 ㎍/㎖) | 2.5 ㎕ | 7.5 ㎕ | 250 ㎕ |
| 1,000X (50 ㎍/㎖) | 5 ㎕ | 15㎕ | 500 ㎕ |
| 500X (100 ㎍/㎖) | 10 ㎕ | 30㎕ | 1㎖ |
a. Myco-Guard™ PLUS solution (50 ㎎/㎖) is diluted to 1:1,000 in culture media.
b. Cultivate Myco-Guard ™ PLUS at a dilution of 1:1,000 media every 3-4 days for 2-3 weeks. It can be used in combination with penicillin and streptomycin.
c. If the cytotoxicity of Myco-Guard™ (50μg/㎖) is high, decrease the concentration (25μg/㎖).
d. After using Myco-Guard™ PLUS solution for 2 weeks in cell cultures, confirm the presence or absence of mycoplasma by PCR or a cell-based method.
※ Confirm the elimination of mycoplasmas by using a mycoplasma PCR detection kit. (SMD0171, Myco-read™)
e. If mycoplasma detection assay is not successful, increase the concentration for one week (75μg/㎖) and treat for one more week.
Note. Observe the cell state daily with a microscope.



